Multi-cancer early detection (MCED) represents a major advancement in cancer screening, using a single blood test to identify signals associated with dozens of different cancers. Unlike traditional screenings that target one type of cancer at a time, MCED tests such as the Galleri, Carcimun, and CancerSEEK tests can detect a wide range of cancers before symptoms arise. By analyzing biomarkers like tumor DNA or protein changes in the blood, these tests enable earlier intervention, which can lead to more effective treatment and improved outcomes. While MCED tests are not diagnostic, their role in early detection is significant, though the cost, ranging from $700 to $950 per test, is currently not widely covered by insurance.
Key Takeaways
- Multi-Cancer Early Detection (MCED) tests enable early detection of various cancers from a single blood sample, improving patient outcomes and survival rates.
- Examples of MCED tests include the GRAIL Galleri Test and the Carcimun Test, which analyze DNA fragments and plasma proteins, respectively, to identify cancer signals.
- MCED tests can be costly and are often not covered by insurance, but their ability to detect multiple cancers early can justify the expense and reduce long-term healthcare costs.
What is a multi-cancer early detection test?
A Multi-Cancer Early Detection (MCED) test is an advanced diagnostic tool that detects the presence of multiple types of cancer using a single blood sample, often before any symptoms appear. These tests work by analyzing biological signals, such as circulating tumor DNA (ctDNA), which are shed by cancer cells into the bloodstream. This allows physicians to identify cancer at its earliest and most treatable stages.
The primary purpose of an MCED test is early cancer detection. By identifying malignancies in their initial stages, these tests enable faster medical intervention, better treatment planning, and significantly improved survival rates.
The term “multi-cancer” reflects the test's ability to screen for a broad spectrum of cancers at once, rather than focusing on just one type. MCED tests can detect over 50 types of cancers, including both common and hard-to-detect forms. These include:
- Common cancers: breast, prostate, lung, and colorectal
- Aggressive and less-screened cancers: pancreatic, ovarian, liver, esophageal, stomach, and head and neck cancers
This wide-ranging capability makes MCED tests a potential complement to standard screening procedures such as mammograms, colonoscopies, and PSA tests, filling critical gaps in early detection efforts for cancers that often go undiagnosed until later stages.
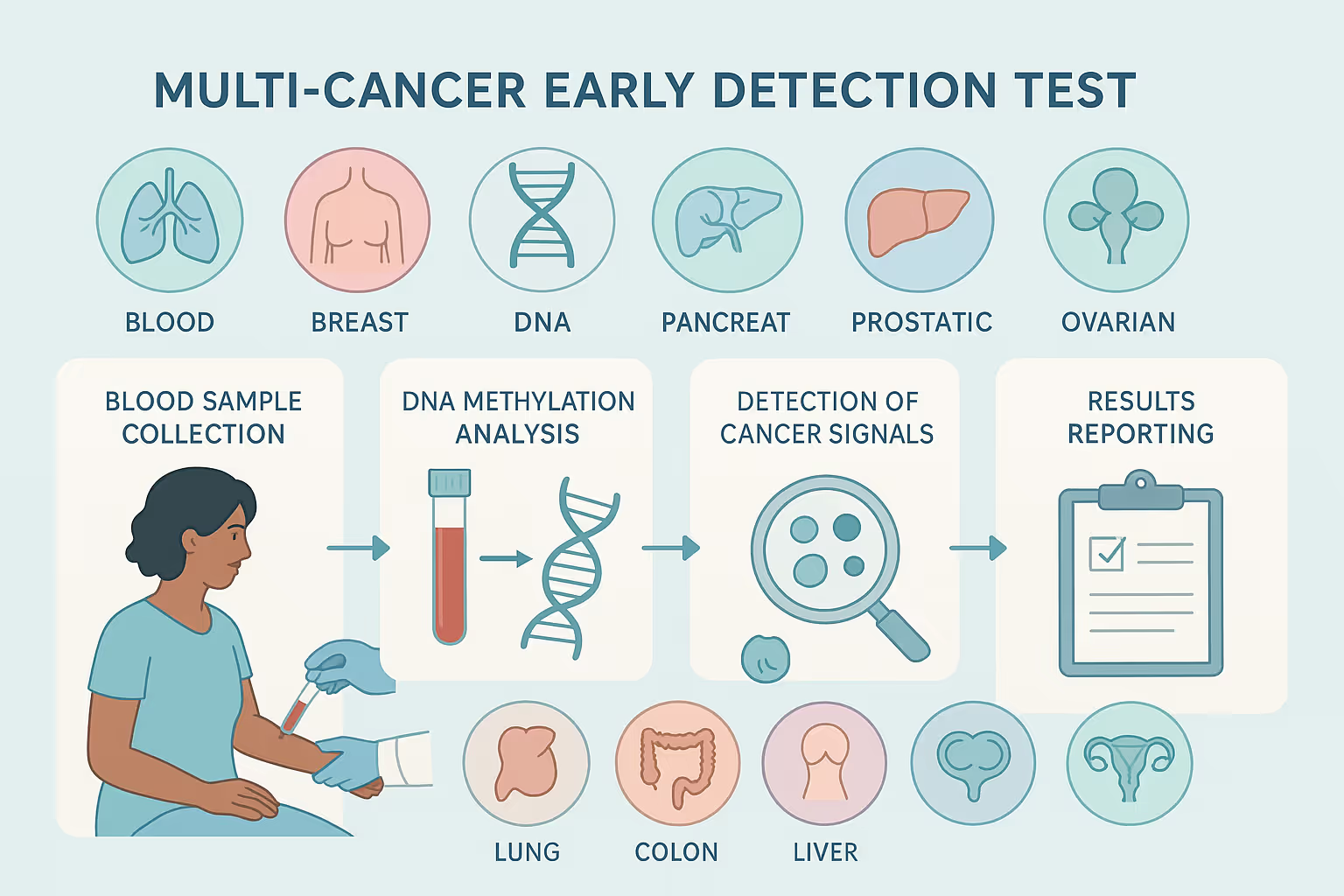
What types of cancer can be detected with multi-cancer early detection tests?
Multi-cancer early detection (MCED) tests can identify a wide range of cancers from a single test for cancer detection. These tests are designed to recognize both common and hard-to-detect cancers by analyzing DNA fragments shed into the bloodstream.
Some of the cancers that MCED tests can detect include breast, prostate, lung, and colorectal cancers, which are among the most prevalent. They are also capable of identifying cancers that are more challenging to screen for through traditional methods, such as pancreatic, ovarian, liver, esophageal, and stomach cancers. Additional types include head and neck cancers, gallbladder cancer, bladder cancer, lymphomas, multiple myeloma, and bile duct cancer.
Despite their broad detection capabilities, MCED tests are not foolproof. Certain cancers may not shed enough detectable DNA into the bloodstream, making them harder to identify through blood-based testing alone. As a result, these tests should complement, not replace, standard screening methods to ensure a thorough and accurate cancer detection strategy.
Are MCED tests for every individual?
No, multi-cancer early detection (MCED) tests are not currently recommended for every individual. These tests are primarily intended for adults at an elevated risk of developing cancer, especially those aged 50 and older or those with a family history of cancer, known genetic predispositions, or other risk factors. This is because the likelihood of developing cancer increases with age, and early detection is most beneficial in populations with higher baseline risk.
For average-risk individuals, routine MCED testing may not be necessary or cost-effective, especially since the tests are not yet fully validated for widespread population screening. Additionally, MCED tests can produce false positives or false negatives, which could lead to unnecessary anxiety, further invasive testing, or missed diagnoses if relied upon alone.
As research progresses and guidelines evolve, recommendations may expand. For now, MCED tests are best used in combination with standard screening protocols and under the guidance of a healthcare provider.
Personalized MCED Testing at Fountain Life
At Fountain Life, we make early cancer detection more precise by offering cutting-edge MCED testing tailored to your unique risk profile. Our precision diagnostics are designed for those who want to take control of their health with proactive, individualized insights. Whether you’re over 50 or have a family history of cancer, our care team helps you determine if an MCED test is right for you, supported by advanced technology and expert medical guidance.
What screening tests are used to detect multiple cancers early?
Multi-cancer early detection (MCED) tests are specialized screening tools that identify DNA or protein-based signals released by tumors into the bloodstream, often before symptoms begin. These tests are designed to detect multiple types of cancer simultaneously through a single blood sample, offering a more comprehensive approach to early cancer detection. Below are some of the leading MCED tests currently in use:
- GRAIL Galleri Test: Uses next-generation sequencing and machine learning to analyze tumor-derived cell-free DNA. It can detect signals from over 50 types of cancer and predict the tissue of origin.
- Carcimun Test: Focuses on detecting changes in plasma proteins. It shows high accuracy in differentiating between cancer patients, healthy individuals, and those with inflammatory conditions.
- PanSeer Test: Identifies DNA methylation patterns associated with cancer. It has shown promise in detecting cancers like liver, stomach, and esophageal cancer before clinical symptoms emerge.
- CancerSEEK Test: Combines the detection of genetic mutations and protein biomarkers. It has demonstrated high sensitivity and specificity in clinical trials, particularly for cancers such as ovarian and pancreatic.
Each test has its own strengths, methodologies, and limitations, and they are often used alongside conventional screening methods for a more complete cancer detection strategy.

1. GRAIL Galleri test
The GRAIL Galleri test is a leading multi-cancer early detection (MCED) test that uses next-generation sequencing and machine learning to analyze cell-free DNA (cfDNA) fragments shed by tumors into the bloodstream. It is designed to identify more than 50 types of cancer, many of which lack standard screening protocols.
The test has shown high specificity (over 99%) and promising sensitivity, especially for detecting cancers that are typically harder to diagnose early. While it is among the most comprehensive MCED tests available, it is not considered a replacement for traditional screenings like mammograms or colonoscopies, it is best used as a supplementary test.
The Galleri test is effective in detecting cancers such as breast, colorectal, lung, liver, pancreatic, ovarian, and head and neck cancers. However, it may not detect cancers that do not release enough cfDNA into the bloodstream or cancers in very early stages. Some hematologic malignancies and early-stage prostate cancers, for example, may go undetected.
According to the current guidelines, the Galleri test is recommended for adults aged 50 and above or younger individuals with elevated cancer risk due to genetic or family history. The process involves a simple blood draw that is sent to a certified laboratory for analysis.
The test is typically recommended once a year for individuals at risk. The cost of the Galleri test is approximately $950 and is not widely covered by insurance as of now.
2. Carcimun test
The Carcimun test is another promising MCED option that detects specific protein markers in plasma. Instead of analyzing DNA, it focuses on identifying patterns in plasma proteins that may signal the presence of cancer. This makes it a useful tool for detecting early-stage cancers using a non-genomic approach.
In clinical studies, the Carcimun test has shown an accuracy rate of 95.4%, effectively distinguishing between cancer patients, healthy individuals, and those with inflammatory diseases. However, it is not considered the primary MCED test on the market due to its more limited clinical adoption and lower availability compared to the Galleri test.
The Carcimun test has shown strong performance in detecting cancers such as lung, colorectal, and pancreatic cancer. However, it can produce false positives in individuals with inflammatory conditions like pneumonia or sarcoidosis, which can mimic cancer-related protein changes. It does not localize tumors and is less effective in detecting cancers with minimal protein changes in early stages.
There are no universally established guidelines for who should take the Carcimun test, but it is generally suited for adults at moderate to high risk of cancer. The procedure involves a standard blood draw, followed by protein analysis in a lab.
Annual testing is considered appropriate for at-risk populations. The cost of the Carcimun test varies depending on the provider and is not yet broadly covered by insurance plans.
3. PanSeer test
The PanSeer test is a research-stage MCED tool that identifies DNA methylation patterns in plasma to detect cancer-related epigenetic changes. It is notable for being one of the few tests that can detect cancer up to four years before clinical symptoms appear.
While still under investigation, PanSeer has demonstrated strong potential in identifying cancers such as liver, stomach, colorectal, esophageal, and lung cancers. It is not considered a primary or widely available MCED test yet, but it has gained attention for its ability to identify cancers that lack routine screening options.
Its effectiveness in detecting asymptomatic cancers makes it a valuable early detection method, although it may not detect cancers that do not involve significant methylation changes or those that progress rapidly. Guidelines for its use are still evolving, as the test is not yet FDA-approved for general clinical use.
PanSeer is primarily intended for research and high-risk groups, especially in controlled study environments. The test is performed via a standard blood sample, and frequency recommendations are not yet standardized due to its pre-commercial stage.
The cost of the PanSeer test has not been publicly disclosed due to its limited clinical availability.
4. CancerSEEK test
CancerSEEK is a multi-analyte MCED test that combines the analysis of DNA mutations and protein biomarkers in the blood to detect several cancer types simultaneously. It was developed to offer a balance of genomic and proteomic insights in a single test.
In clinical trials, CancerSEEK has shown sensitivity ranging from 69% to 98% for certain cancer types, with high specificity across all tested samples. It is not yet the most widely used MCED test, but it has strong scientific support and is progressing toward broader clinical use.
CancerSEEK has demonstrated effectiveness in detecting ovarian, pancreatic, liver, and esophageal cancers, many of which do not have effective early screening options. However, it may be less effective for cancers with fewer genetic mutations or low biomarker expression, such as some early-stage or blood cancers.
While formal guidelines are still pending, CancerSEEK is primarily intended for use in adults over 50 or those with a high-risk cancer profile. The test requires a standard blood draw and lab processing to analyze the mutation and protein patterns.
Like other MCED tests, CancerSEEK is recommended annually for eligible individuals. Pricing details are not widely published, as the test is still in pre-commercial or early clinical use phases in many regions.
How frequently should the MCED test be done?
MCED tests are generally recommended once every 12 months (annually) for individuals at elevated risk of cancer, such as adults aged 50 and older or those with a family history of cancer. This yearly interval helps detect potential cancers at an early, more treatable stage while minimizing unnecessary testing.
Currently, annual testing strikes a balance between effectiveness and resource use. However, the exact frequency may vary based on personal risk factors and emerging research. As clinical guidelines continue to evolve, your healthcare provider may adjust the recommended frequency based on your individual health profile.
How do MCED tests help in the early detection of multiple cancers?
MCED tests support cancer early detection by identifying biological signals from tumors, such as circulating DNA fragments, before symptoms appear. These tests analyze a single blood sample to detect multiple types of cancer at once, allowing for a broader and earlier assessment than traditional, cancer-specific screening methods.
By catching cancers in their early stages, when they are often more localized and easier to treat, MCED tests improve the likelihood of successful treatment outcomes. This proactive approach reduces the need for more aggressive interventions later and has the potential to significantly lower cancer-related mortality across various cancer types.
Can a blood test detect multiple cancers in the body?
Yes, blood tests like the Galleri test can detect multiple types of cancer simultaneously. These tests analyze circulating DNA shed by tumors into the bloodstream, identifying unique biomarkers associated with different cancers. This capability allows for the detection of various cancers from a single blood sample, making it a powerful tool in early cancer detection.
However, it is important to note that not all cancers produce detectable levels of these biomarkers, and false positives and false negatives are possible. Thus, MCED tests should complement traditional screening methods for thorough cancer detection.
Are there different blood work results for different cancers?
Yes, different cancers produce distinct biological markers that result in different blood work profiles. Each type of cancer can release unique substances into the bloodstream, such as tumor DNA fragments, proteins, or other biomarkers. For example, prostate cancer often elevates prostate-specific antigen (PSA) levels, while ovarian cancer may be associated with increased CA-125 levels. MCED tests leverage this principle by analyzing patterns in cell-free DNA (cfDNA) or plasma proteins specific to different cancers.
The GRAIL Galleri test, for instance, uses methylation patterns in cfDNA to determine both the presence of cancer and the likely tissue of origin. Similarly, the CancerSEEK test combines genetic mutations and protein biomarkers to differentiate between multiple cancer types. These differences in molecular signals form the basis for detecting and identifying various cancers through blood-based testing.
How much does a multi-cancer early detection test cost?
The cost of a multi-cancer early detection (MCED) test typically ranges from $700 to $950, depending on the test type, healthcare provider, and location. For example, the GRAIL Galleri test, one of the most well-known MCED tests, is priced at approximately $950.
This cost includes the collection of a blood sample, laboratory analysis of circulating tumor DNA (ctDNA) or protein biomarkers, and the use of advanced machine learning algorithms to interpret the results. Several factors can influence the final cost of the test, including:
- The technology and methodology used (e.g., DNA sequencing vs. protein analysis)
- The number of cancers screened
- Clinical setting or provider fees
- Follow-up diagnostics if a cancer signal is detected
While MCED tests are relatively expensive, they offer potential long-term savings by enabling earlier, less invasive treatment and reducing the costs associated with late-stage cancer care.

Invest in Your Future Health with Fountain Life
While MCED tests may seem costly upfront, Fountain Life helps make them a powerful investment in your long-term health. We offer access to advanced early detection technologies in combination with comprehensive health assessments, enabling you to identify risks before they become life-threatening. With Fountain Life, you gain more than just a test, you gain a plan for longevity and optimal wellness.
Are MCED tests covered by insurance?
No, multi-cancer early detection (MCED) tests are generally not covered by most health insurance plans at this time. Individuals typically bear the full cost out of pocket. For instance, the Galleri test by GRAIL is priced at approximately $949 and is not widely reimbursed by insurers.
However, there are exceptions. Some insurance providers have begun to include MCED tests in their coverage. For example, Curative Insurance Company offers the Galleri test as a benefit with no copay or deductible for eligible members. Additionally, TRICARE covers the Galleri test for eligible beneficiaries, subject to prior authorization.
Legislative efforts are underway to expand insurance coverage for MCED tests. The proposed Multi-Cancer Early Detection Screening Coverage Act aims to establish a benefit category under Medicare, facilitating coverage for these tests upon FDA approval.
What are the benefits of MCED Tests?
Multi-cancer early detection (MCED) tests offer a wide range of advantages, particularly for individuals at higher risk of developing cancer. These tests enhance existing cancer screening methods and provide a more proactive approach to health monitoring. Key benefits include:
- Comprehensive detection: Identifies multiple types of cancer from a single blood sample, including those that lack standard screening tests.
- Early diagnosis: Detects cancers at earlier stages when treatment is more effective and survival rates are higher.
- Minimally invasive: Requires only a simple blood draw, offering a convenient alternative to more invasive procedures like biopsies or colonoscopies.
- Fills screening gaps: Screens for cancers such as pancreatic, ovarian, and liver cancers, which are difficult to detect early through conventional means.
- Improves outcomes: Early intervention based on MCED results can reduce cancer-related mortality and improve quality of life.
- Supports risk-based screening: Especially valuable for adults aged 50+ or those with family history or genetic predisposition to cancer.
- Potential cost efficiency: By catching cancer early, MCED testing may reduce the need for more costly, late-stage cancer treatments.
These benefits position MCED tests as a transformative addition to modern preventive healthcare strategies.
Are MCED tests used to detect cancer?
Yes, multi-cancer early detection (MCED) tests are specifically designed to detect cancer signals in the body, often before symptoms arise. They analyze biomarkers like circulating tumor DNA (ctDNA) or abnormal protein patterns in the blood to identify the possible presence of cancer. For example, the Galleri test detects over 50 types of cancer by analyzing DNA methylation patterns from a single blood sample. However, detection does not confirm diagnosis, it only indicates the potential presence of cancer for further investigation.
Are MCED tests used to diagnose cancer?
No, MCED tests are not diagnostic tools. While they can signal the possible presence of cancer, a formal diagnosis requires follow-up procedures such as imaging, biopsies, or histopathological analysis to confirm the type, location, and stage of the disease. For instance, if the Galleri test detects a cancer signal, a physician will recommend additional tests to establish a definitive diagnosis.
Are MCED tests used to treat cancer?
No, MCED tests do not play a role in treating cancer. Their function is limited to early detection and risk assessment. Treatment decisions, such as surgery, chemotherapy, or immunotherapy, are based on diagnostic confirmation and clinical evaluation, not on MCED results alone. MCED tests are valuable in guiding further investigation, but they are not therapeutic tools.